For healthcare professionals

Evidence of variation: active content and degradation, impacting efficacy
Studies have shown that generic eyedrops:
- Have an inferior therapeutic profile to the originator brand with up to 20% difference in administered dose.4
- Have differences in drop size and drop volume.1
This has an impact on efficacy:
- There is risk of losing control of the patient's IOP.4
- Higher IOP has been recorded when patients were dispensed generics.5
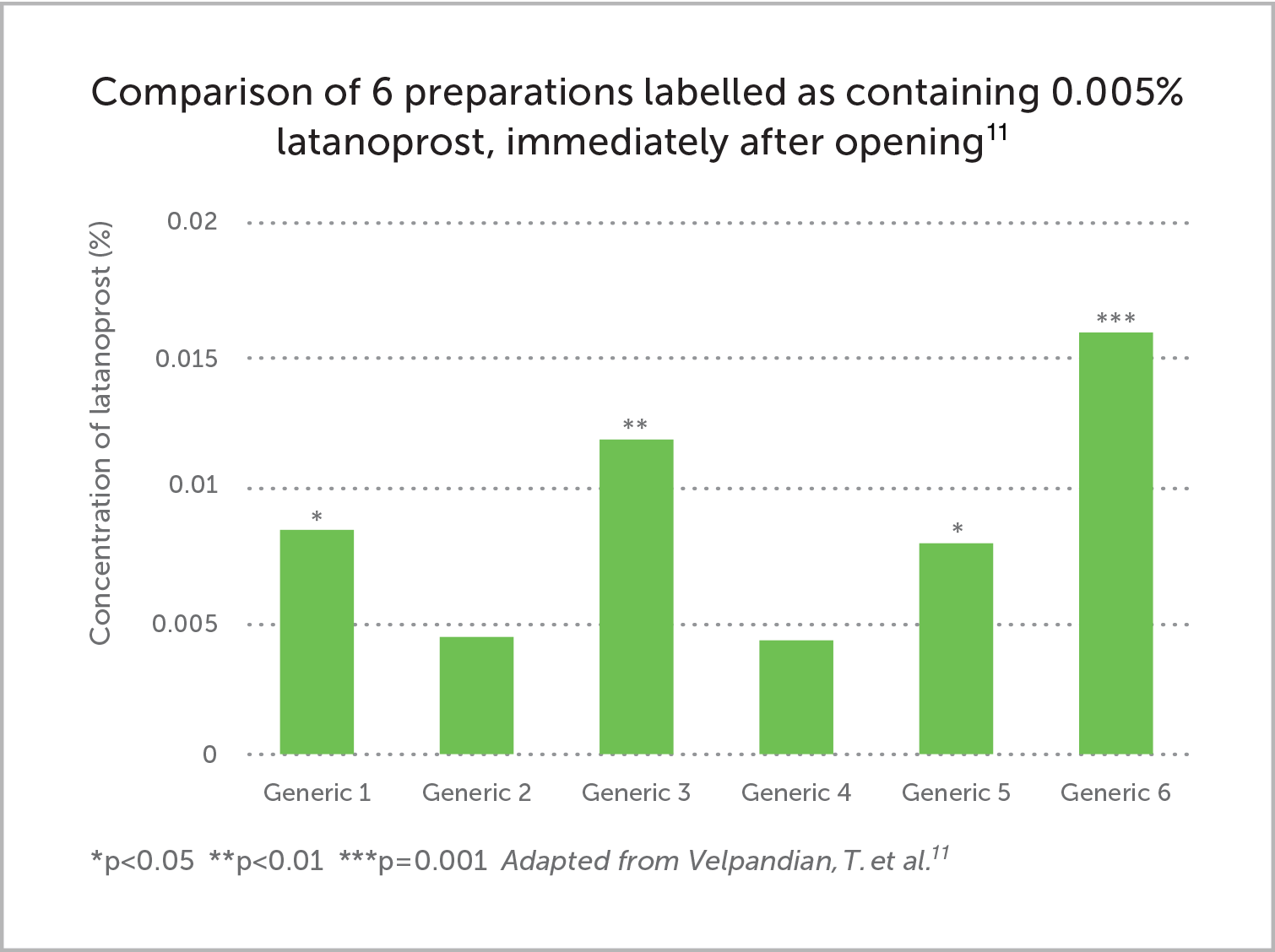
A study of 6 latanoprost generics found a variation in active content of 90% to 330% when first opening, compared to the label claim. This reduced to 20% to 250% after 30 days of `simulated patient usage'.11